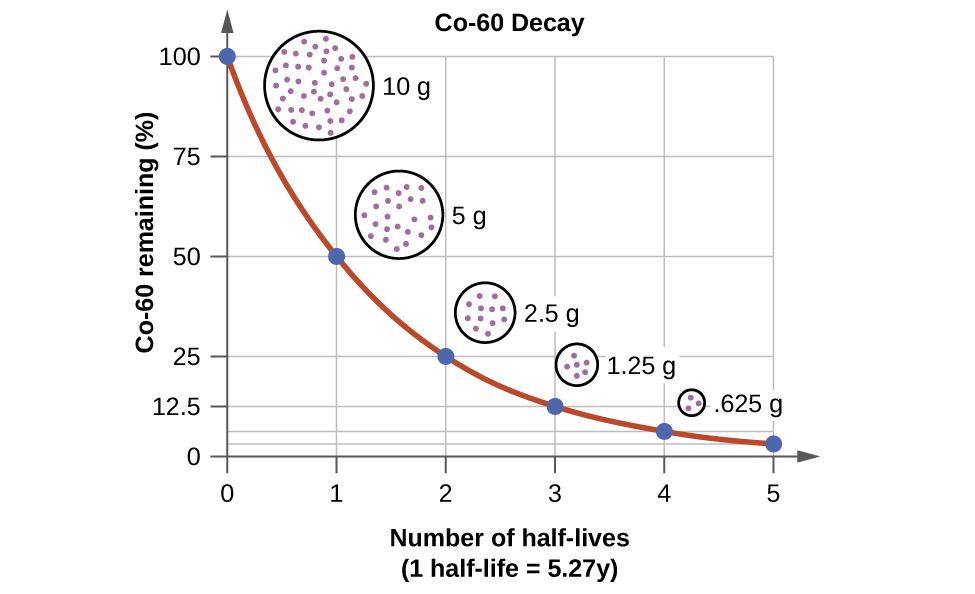
What Is Meant By The Half-Life Of A Radioactive Substance . As you can see, the. for any substantial sample of a radioactive isotope, half the atoms will decay in a period known as that isotope’s half. The amount of the radioactive substance present, and. when a radioactive element decays, the number of radioactive nuclei decreases with time. In this first chart, we have a radioactive substance with a half life of 5 years. For example, if the starting concentration of a. it represents the time for half of a given quantity of a substance to transform into something else. half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. intuitively, you would expect the activity of a source to depend on two things: The time it takes for the number of. Calculate the amount of radioactive. Calculate the amount of radioactive material that will remain.
from chem.libretexts.org
intuitively, you would expect the activity of a source to depend on two things: for any substantial sample of a radioactive isotope, half the atoms will decay in a period known as that isotope’s half. when a radioactive element decays, the number of radioactive nuclei decreases with time. The amount of the radioactive substance present, and. The time it takes for the number of. Calculate the amount of radioactive material that will remain. In this first chart, we have a radioactive substance with a half life of 5 years. it represents the time for half of a given quantity of a substance to transform into something else. Calculate the amount of radioactive. For example, if the starting concentration of a.
14.3 Radioactivity and HalfLife Chemistry LibreTexts
What Is Meant By The Half-Life Of A Radioactive Substance it represents the time for half of a given quantity of a substance to transform into something else. half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. The time it takes for the number of. For example, if the starting concentration of a. Calculate the amount of radioactive. As you can see, the. it represents the time for half of a given quantity of a substance to transform into something else. intuitively, you would expect the activity of a source to depend on two things: for any substantial sample of a radioactive isotope, half the atoms will decay in a period known as that isotope’s half. The amount of the radioactive substance present, and. Calculate the amount of radioactive material that will remain. when a radioactive element decays, the number of radioactive nuclei decreases with time. In this first chart, we have a radioactive substance with a half life of 5 years.
From pediaa.com
Relationship Between Radioactive Decay and Half Life Definition What Is Meant By The Half-Life Of A Radioactive Substance Calculate the amount of radioactive. The amount of the radioactive substance present, and. when a radioactive element decays, the number of radioactive nuclei decreases with time. For example, if the starting concentration of a. intuitively, you would expect the activity of a source to depend on two things: As you can see, the. half life is the. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.chegg.com
Solved 2. The halflife of a radioactive substance is 4 What Is Meant By The Half-Life Of A Radioactive Substance The time it takes for the number of. As you can see, the. half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. The amount of the radioactive substance present, and. In this first chart, we have a radioactive substance with a half life of 5. What Is Meant By The Half-Life Of A Radioactive Substance.
From ar.inspiredpencil.com
Radioactive Isotope Half Life What Is Meant By The Half-Life Of A Radioactive Substance As you can see, the. for any substantial sample of a radioactive isotope, half the atoms will decay in a period known as that isotope’s half. it represents the time for half of a given quantity of a substance to transform into something else. In this first chart, we have a radioactive substance with a half life of. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.slideserve.com
PPT Radioactive Isotopes and Half Life PowerPoint Presentation, free What Is Meant By The Half-Life Of A Radioactive Substance when a radioactive element decays, the number of radioactive nuclei decreases with time. intuitively, you would expect the activity of a source to depend on two things: half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. for any substantial sample of a. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.slideserve.com
PPT Radioactivity PowerPoint Presentation, free download ID36026 What Is Meant By The Half-Life Of A Radioactive Substance Calculate the amount of radioactive. when a radioactive element decays, the number of radioactive nuclei decreases with time. The amount of the radioactive substance present, and. for any substantial sample of a radioactive isotope, half the atoms will decay in a period known as that isotope’s half. intuitively, you would expect the activity of a source to. What Is Meant By The Half-Life Of A Radioactive Substance.
From chem.libretexts.org
HalfLives and Radioactive Decay Chemistry LibreTexts What Is Meant By The Half-Life Of A Radioactive Substance For example, if the starting concentration of a. As you can see, the. when a radioactive element decays, the number of radioactive nuclei decreases with time. Calculate the amount of radioactive. The time it takes for the number of. half life is the time that it takes for half of the original value of some amount of a. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.youtube.com
Halflife of Radioactive Substance Example YouTube What Is Meant By The Half-Life Of A Radioactive Substance Calculate the amount of radioactive. Calculate the amount of radioactive material that will remain. it represents the time for half of a given quantity of a substance to transform into something else. when a radioactive element decays, the number of radioactive nuclei decreases with time. For example, if the starting concentration of a. The time it takes for. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.slideshare.net
RADIOACTIVE DECAY AND HALFLIFE CONCEPTS What Is Meant By The Half-Life Of A Radioactive Substance The amount of the radioactive substance present, and. The time it takes for the number of. when a radioactive element decays, the number of radioactive nuclei decreases with time. Calculate the amount of radioactive. Calculate the amount of radioactive material that will remain. it represents the time for half of a given quantity of a substance to transform. What Is Meant By The Half-Life Of A Radioactive Substance.
From brainly.com
📈A radioactive substance has a halflife of 7 hours. If a sample of the What Is Meant By The Half-Life Of A Radioactive Substance Calculate the amount of radioactive material that will remain. The amount of the radioactive substance present, and. For example, if the starting concentration of a. it represents the time for half of a given quantity of a substance to transform into something else. half life is the time that it takes for half of the original value of. What Is Meant By The Half-Life Of A Radioactive Substance.
From mmerevise.co.uk
Radioactive Halflife Worksheets, Questions and Revision MME What Is Meant By The Half-Life Of A Radioactive Substance it represents the time for half of a given quantity of a substance to transform into something else. In this first chart, we have a radioactive substance with a half life of 5 years. The time it takes for the number of. for any substantial sample of a radioactive isotope, half the atoms will decay in a period. What Is Meant By The Half-Life Of A Radioactive Substance.
From ukinventory.nda.gov.uk
What is radiation? UK Radioactive Waste Inventory (UKRWI) What Is Meant By The Half-Life Of A Radioactive Substance half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. Calculate the amount of radioactive. For example, if the starting concentration of a. The time it takes for the number of. intuitively, you would expect the activity of a source to depend on two things:. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.youtube.com
Halflife of a radioactive substance A is two times the halflife of What Is Meant By The Half-Life Of A Radioactive Substance it represents the time for half of a given quantity of a substance to transform into something else. Calculate the amount of radioactive. when a radioactive element decays, the number of radioactive nuclei decreases with time. The time it takes for the number of. For example, if the starting concentration of a. intuitively, you would expect the. What Is Meant By The Half-Life Of A Radioactive Substance.
From haipernews.com
How To Calculate Half Life In Years Haiper What Is Meant By The Half-Life Of A Radioactive Substance As you can see, the. half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. when a radioactive element decays, the number of radioactive nuclei decreases with time. For example, if the starting concentration of a. Calculate the amount of radioactive material that will remain.. What Is Meant By The Half-Life Of A Radioactive Substance.
From dxolokulm.blob.core.windows.net
Half Life Equation Step 1 at Albert Duffy blog What Is Meant By The Half-Life Of A Radioactive Substance half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. The time it takes for the number of. The amount of the radioactive substance present, and. when a radioactive element decays, the number of radioactive nuclei decreases with time. for any substantial sample of. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.youtube.com
HalfLife Formula and Calculation Understanding Radioactive Decay What Is Meant By The Half-Life Of A Radioactive Substance when a radioactive element decays, the number of radioactive nuclei decreases with time. Calculate the amount of radioactive material that will remain. The time it takes for the number of. In this first chart, we have a radioactive substance with a half life of 5 years. it represents the time for half of a given quantity of a. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.toppr.com
The half life of a radioactive substance is 20 minutes. The time What Is Meant By The Half-Life Of A Radioactive Substance intuitively, you would expect the activity of a source to depend on two things: As you can see, the. it represents the time for half of a given quantity of a substance to transform into something else. The time it takes for the number of. Calculate the amount of radioactive. for any substantial sample of a radioactive. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.youtube.com
The half life of a radioactive substance is 30 days. What is the time What Is Meant By The Half-Life Of A Radioactive Substance intuitively, you would expect the activity of a source to depend on two things: The time it takes for the number of. For example, if the starting concentration of a. In this first chart, we have a radioactive substance with a half life of 5 years. Calculate the amount of radioactive. As you can see, the. when a. What Is Meant By The Half-Life Of A Radioactive Substance.
From www.toppr.com
If half life of a radioactive substance is 60 minutes, then the What Is Meant By The Half-Life Of A Radioactive Substance when a radioactive element decays, the number of radioactive nuclei decreases with time. half life is the time that it takes for half of the original value of some amount of a radioactive element to decay. The amount of the radioactive substance present, and. As you can see, the. Calculate the amount of radioactive material that will remain.. What Is Meant By The Half-Life Of A Radioactive Substance.